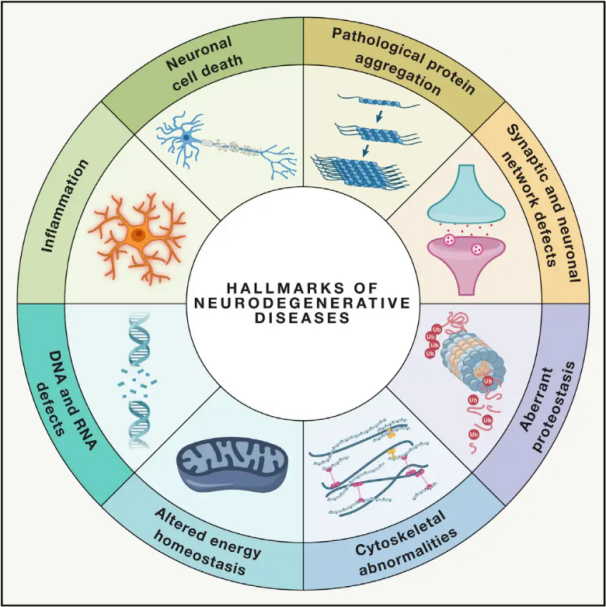
Neurodegenerative diseases (NDDs) are characterized by the progressive or persistent loss of specific vulnerable neuronal populations within the brain or spinal cord. The classification of NDDs can be based on various criteria, including the anatomical distribution of neurodegeneration (such as extrapyramidal disorders, frontotemporal degeneration, or spinocerebellar ataxias), primary molecular abnormalities (like amyloid-β, prions, tau, or α-synuclein), or major clinical features (such as Parkinson's disease, amyotrophic lateral sclerosis, and dementia). Despite these differences in classification and symptom presentation, disorders such as Parkinson’s Disease (PD), Amyotrophic Lateral Sclerosis (ALS), and Alzheimer’s Disease (AD) share common underlying processes leading to neuronal dysfunction and eventual cell death.
With millions worldwide affected by NDDs, the World Health Organization estimates that by 2040, these diseases will become the second leading cause of death in developed countries. While there are various treatments available to alleviate and manage the symptoms associated with specific diseases, effective methods to slow down or cure the progression of these conditions remain elusive. Recent studies indicate a shift in treatment paradigms from mere symptomatic management to utilizing cell protective mechanisms to prevent further deterioration. Extensive evidence suggests that oxidative stress and inflammation play pivotal roles in neurodegeneration, positioning these mechanisms as critical targets for cellular protection. In recent years, foundational and clinical research has unveiled the potential of Hyperbaric Oxygen Therapy (HBOT) in treating neurodegenerative diseases
Understanding Hyperbaric Oxygen Therapy (HBOT)
HBOT typically involves increasing pressure to above 1 absolute atmosphere (ATA) — the pressure at sea level — for a duration of 90-120 minutes, often requiring multiple sessions depending on the specific condition being treated. The enhanced air pressure improves the delivery of oxygen to cells, which in turn stimulates stem cell proliferation and enhances the healing processes mediated by certain growth factors.
Originally, the application of HBOT was founded on the Boyle-Marriott law, which posits the pressure-dependent reduction of gas bubbles, alongside the benefits of high oxygen levels in tissues. There is a range of pathologies known to benefit from the hyperoxic state produced by HBOT, including necrotic tissues, radiation injuries, trauma, burns, compartment syndrome, and gas gangrene, among others listed by the Undersea and Hyperbaric Medical Society. Notably, HBOT has also shown efficacy as an adjunct treatment in various inflammatory or infectious disease models, such as colitis and sepsis. Given its anti-inflammatory and oxidative mechanisms, HBOT offers significant potential as a therapeutic avenue for neurodegenerative diseases.
Preclinical Studies of Hyperbaric Oxygen Therapy in Neurodegenerative Diseases: Insights from the 3×Tg Mouse Model
One of the notable studies focused on the 3×Tg mouse model of Alzheimer’s disease (AD), which showcased the therapeutic potential of HBOT in ameliorating cognitive deficits. The research involved 17-month-old male 3×Tg mice compared to 14-month-old male C57BL/6 mice serving as controls. The study demonstrated that HBOT not only improved cognitive function but also significantly reduced inflammation, plaque load, and Tau phosphorylation—a critical process associated with AD pathology.
The protective effects of HBOT were attributed to a decrease in neuroinflammation. This was evidenced by the reduction of microglial proliferation, astrogliosis, and the secretion of pro-inflammatory cytokines. These findings emphasize the dual role of HBOT in enhancing cognitive performance while simultaneously mitigating neuroinflammatory processes associated with Alzheimer’s disease.
Another preclinical model utilized 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice to evaluate the protective mechanisms of HBOT on neuronal function and motor capabilities. The results indicated that HBOT contributed to enhanced motor activity and grip strength in these mice, correlating with an increase in mitochondrial biogenesis signaling, specifically through the activation of SIRT-1, PGC-1α, and TFAM. This highlights the significant role of mitochondrial function in the neuroprotective effects of HBOT.
The Mechanisms of HBOT in Neurodegenerative Diseases
The underlying principle of utilizing HBOT for NDDs lies in the relationship between reduced oxygen supply and the susceptibility to neurodegenerative changes. Hypoxia-inducible factor-1 (HIF-1) plays a central role as a transcription factor that enables cellular adaptation to low oxygen tension and has been implicated in various NDDs including AD, PD, Huntington’s Disease, and ALS, marking it as a crucial drug target.
Due to age being a significant risk factor for multiple neurodegenerative disorders, investigating the impact of HBOT on aging neurobiology is vital. Studies have indicated that HBOT can improve age-related cognitive deficits in healthy older subjects. Additionally, elderly patients with significant memory impairments exhibited cognitive improvements and increased cerebral blood flow following exposure to HBOT.
1. Impact of HBOT on Inflammation and Oxidative Stress
HBOT has demonstrated the ability to alleviate neuroinflammation in patients with severe brain dysfunction. It possesses the capacity to downregulate pro-inflammatory cytokines (such as IL-1β, IL-12, TNFα, and IFNγ) while upregulating anti-inflammatory cytokines (like IL-10). Some researchers propose that reactive oxygen species (ROS) generated by HBOT mediate several beneficial effects of the therapy. Consequently, apart from its pressure-dependent bubble-reducing action and the attainment of high tissue oxygen saturation, the positive outcomes linked to HBOT are partly dependent on the physiological roles of the produced ROS.
2. Effects of HBOT on Apoptosis and Neuroprotection
Research has indicated that HBOT can reduce hippocampal phosphorylation of p38 mitogen-activated protein kinase (MAPK), subsequently improving cognition and lessening hippocampal damage. Both standalone HBOT and in combination with Ginkgo biloba extract have been found to lower the expression of Bax and the activity of caspase-9/3, resulting in decreased apoptosis rates in rodent models induced by aβ25-35. Furthermore, another study demonstrated that HBOT preconditioning induced tolerance against cerebral ischemia, with mechanisms involving increased SIRT1 expression, alongside augmented B-cell lymphoma 2 (Bcl-2) levels and reduced active caspase-3, underscoring HBOT’s neuroprotective and anti-apoptotic properties.
3. Influence of HBOT on Circulation and Neurogenesis
Exposing subjects to HBOT has been associated with multiple effects on the cranial vascular system, including enhancing blood-brain barrier permeability, promoting angiogenesis, and reducing edema. In addition to providing increased oxygen supplies to tissues, HBOT fosters vascular formation by activating transcription factors like vascular endothelial growth factor and by stimulating the proliferation of neural stem cells.
4. Epigenetic Effects of HBOT
Studies have revealed that exposure of human microvascular endothelial cells (HMEC-1) to hyperbaric oxygen significantly regulates 8,101 genes, including both upregulated and downregulated expressions, highlighting an increase in gene expression associated with antioxidant response pathways.
Conclusion
The usage of HBOT has made significant strides over time, proving its availability, reliability, and safety in clinical practice. While HBOT has been explored as an off-label treatment for NDDs and some research has been conducted, there remains a pressing need for rigorous studies to standardize HBOT practices in treating these conditions. Further research is essential to determine optimal treatment frequencies and assess the extent of beneficial effects for patients.
In summary, the intersection of hyperbaric oxygen and neurodegenerative diseases demonstrates a promising frontier in therapeutic possibilities, warranting continued exploration and validation in clinical settings.
Post time: May-16-2025

